Abstract
Introduction: Vaso-occlusive pain episodes (VOE) are the leading cause of emergency department (ED) visits & hospitalization in patients with sickle cell disease (SCD). During SCD-VOE, patients develop an acute arginine (Arg) deficiency. Supplementation with Arg has shown opioid-sparing effects, improves blood pressure & cardiopulmonary function & decreases length of hospital stay. Though we have previously demonstrated that intravenous (IV) Arg improves mitochondrial (mito) function, the precise mechanisms by which Arg mediates these positive effects remain unclear.
Objectives: To determine the role of IV Arg as adjuvant in the management of SCD-VOE
Methods: Prospective single-center double-blind randomized controlled trial (RCT) of IV Arg (TID, up to 7 days) in children with SCD age 3-21 years hospitalized for VOE requiring IV opioids. Patients with significant liver/renal dysfunction or those previously enrolled were excluded. Subjects were randomized into 1 of 3 arms: 1) 100 mg/kg/dose Arg, standard dose (SD), 2) loading dose: 200 mg/kg followed by SD or 3) placebo. Demographics, total parenteral opioid (TPO) use (morphine equivalents, mg/kg) time to crisis resolution (time of study drug delivery to last IV opioid in hours), pain scores, patient reported outcomes (PROMIS) & mito function were obtained before treatment & at discharge. The primary outcome measure was TPO use between study arms. Hypothesis tests & unadjusted & covariate-adjusted fixed & mixed effects generalized linear regression models were used, as appropriate, to compare cross-sectional & longitudinal outcomes within & between randomization arms. This protocol utilized IND#66943 (Sponsor-Morris), registered with ClinicalTrials.gov (NCT02536170).
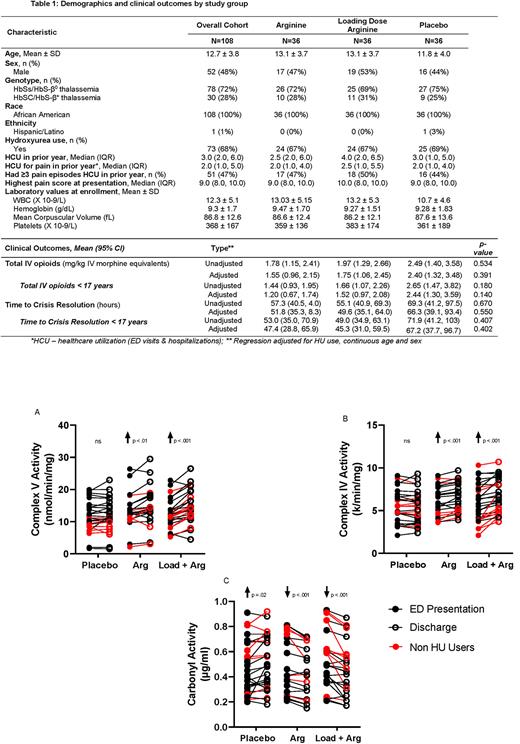
Results: 1,548 patients were screened, 266 were eligible, 114 consented, & 108 were randomized. Safety results of this RCT have been previously reported (Reyes et al, Am J Hematol 2022). Demographics & clinical outcomes of subjects randomized by treatment arm are provided in Table 1. While statistically insignificant, there was a clinically relevant decrease in TPO & time to crisis resolution in both Arg arms compared to placebo. The placebo group required 45% higher TPO & experienced >15 hours longer mean time to crisis resolution compared to combined Arg-treated groups when adjusted for HU use, continuous age and sex. Among children <17 years, the placebo group (n=33) required 80% more TPO compared to combined Arg groups (n=57; p=0.075). No differences in ED vs discharge pain scores or patient/parent PROMIS reports across arms were found. Mito Complex V activity was higher (p=0.02) & protein carbonyl levels were lower (p=0.003) at ED visit in patients on Hydroxyurea (HU). Notably mito Complex IV & V activity increased significantly in both Arg arms while there was no change in the placebo group (Fig 1, p<0.001); protein carbonyl levels in platelet rich plasma decreased in both Arg groups (Fig 1, p<0.001), suggesting a decrease in oxidative stress that increased in the placebo arm (p=0.02). Greatest mito improvement occurred with Arg loading dose.
Conclusion: Arg supplementation increases mito activity & decreases oxidative stress in children with SCD-VOE. This is the first report to suggest a HU-related impact on mito activity in SCD. Prior reports show that Complex V inhibition leads to increased mito oxidant production in platelets from SCD patients. Acute improvements in Complex IV & V function & decreased oxidative markers with Arg therapy are consistent with Arg-induced reductions in mito oxidant generation. Arg therapy improves symptoms associated with MELAS syndrome (mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes) suggesting an impact of Arg on mito activity that may be beneficial in SCD. Given emerging data supporting a link between mito function and pain, improved mito activity may mechanistically contribute to decreased pain & ultimately less opioid requirement with IV Arg treatment, as well as have further implications for improved metabolism and oxidative signaling in SCD. In this RCT, Arg therapy also demonstrated clinically relevant opioid-sparing & a decreased time to crisis resolution that was statistically insignificant. A larger sample size may elucidate potential differences; we are currently enrolling 360 children in an ongoing PECARN-endorsed multicenter Phase 3 RCT (SCD Treatment with Arg Therapy - STArT).
Disclosures
Morris:CSL Behring: Consultancy; UCSF Benioff Children's Hospital Oakland: Patents & Royalties: inventor of IP generating Royalties. Dampier:Pfizer: Consultancy, Other: clinical trial, advisory board, Research Funding; Fulcrum Therapeutics: Consultancy, Other: clinical trial; Merck: Other: clinical trial, Research Funding; NIH/NICHD and NCATS: Research Funding; Global Blood Therapeutics: Consultancy; Hilton Publishing Company: Consultancy; Glycomimetics: Consultancy; Forma Therapeutics: Consultancy; CSL Behring: Consultancy; Syneos: Consultancy, Other: DSMB Chair for Clinical trial.
OffLabel Disclosure:
L-Arginine for treatment of Sickle Cell Disease Vaso-occlusive pain
Author notes
Asterisk with author names denotes non-ASH members.